Automate and centralize financial operations. The system connects production and inventory with accounting for accurate, real-time financial reporting.
Key Features:
• General ledger, AR, AP, cash, bank, budgeting
• Multi-currency support
• Financial consolidation across plants
• Automatic postings from inventory and production
One ERP. Every Industry. Global Presence. Powered by Azaalea AI

One ERP. Every Industry. Global Presence. Powered by
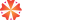
ERP for Pharmaceutical
Industry
eresource Bpro, An ERP system for Pharmaceutical, Bulk Drug & API Manufacturers
ERP for Pharmaceutical Industry
eresource Bpro, An ERP system for Pharmaceutical, Bulk Drug & API Manufacturers
ERP for Pharmaceutical Industry
The pharmaceutical industry works under some of the most demanding regulatory, quality, and safety standards. Manufacturers must handle precise formulations, controlled environments, batch production, validation, documentation, and real-time traceability across every stage from raw material sourcing to final packaging. Managing production schedules, QC testing, equipment sterilization, documentation, and compliance manually increases risk, delays approvals, and reduces overall efficiency.
eresource Bpro ERP is built specifically for pharmaceutical companies, bulk drug manufacturers, API producers, formulation units, and contract manufacturers. It integrates formulation management, batch processing, validation, quality control, warehouse management, supply chain, and finance into a unified, compliant platform.
The system supports tablets, capsules, syrups, injectables, ointments, powders, APIs, intermediates, and bulk drugs. With digital batch records, controlled workflows, and compliance-ready documentation, manufacturers can maintain consistency, meet global regulatory standards, and ensure safe, high-quality production.
With real-time visibility, automated workflows, and strict traceability, eresource Bpro helps companies eliminate manual errors, shorten production cycles, and maintain unmatched regulatory compliance across all operations.
Trusted by Leading Companies




Enquire Now
Fill out the form below to get in touch with us
Why Choose eresource Bpro ERP for the Pharmaceutical Industry?
Pharmaceutical manufacturing requires precise batch control, ingredient-level traceability, validated processes, and adherence to strict guidelines such as FDA, WHO, cGMP, and Schedule M. Manual systems cannot ensure compliance, quality consistency, or data integrity. eresource Bpro ERP brings all production, QC, documentation, and traceability onto one secure platform.
- Key Advantages:
- Manages formulations, batch recipes, and production protocols with accuracy.
- Tracks raw materials, APIs, intermediates, and excipients with full traceability.
- Ensures strict QC with digital lab tests, stability studies, and inspection logs.
- Supports cGMP, FDA, WHO, MHRA, EU-GMP, and Schedule M compliance.
- Manages warehousing with FEFO, temperature control, and restricted access areas.
- Integrates costing, billing, receivables, and payables seamlessly.
With validated processes and controlled workflows, eresource Bpro enables safe, compliant, and efficient pharmaceutical manufacturing.
Core Modules
Formulation & Recipe Management
Manage every aspect of your product formulas with precision. This module helps process industries create, track, and optimize formulations while maintaining strict control over ingredients, variations, and cost impact.
Key Features:
• Multi-level formula creation with percentage or weight/volume inputs
• Alternate raw materials and potency-based adjustments
• Formula scaling for any batch size
• Revision control for formulation changes
• Restricted access for sensitive formulas
• Automatic yield, loss, overhead, and cost calculations
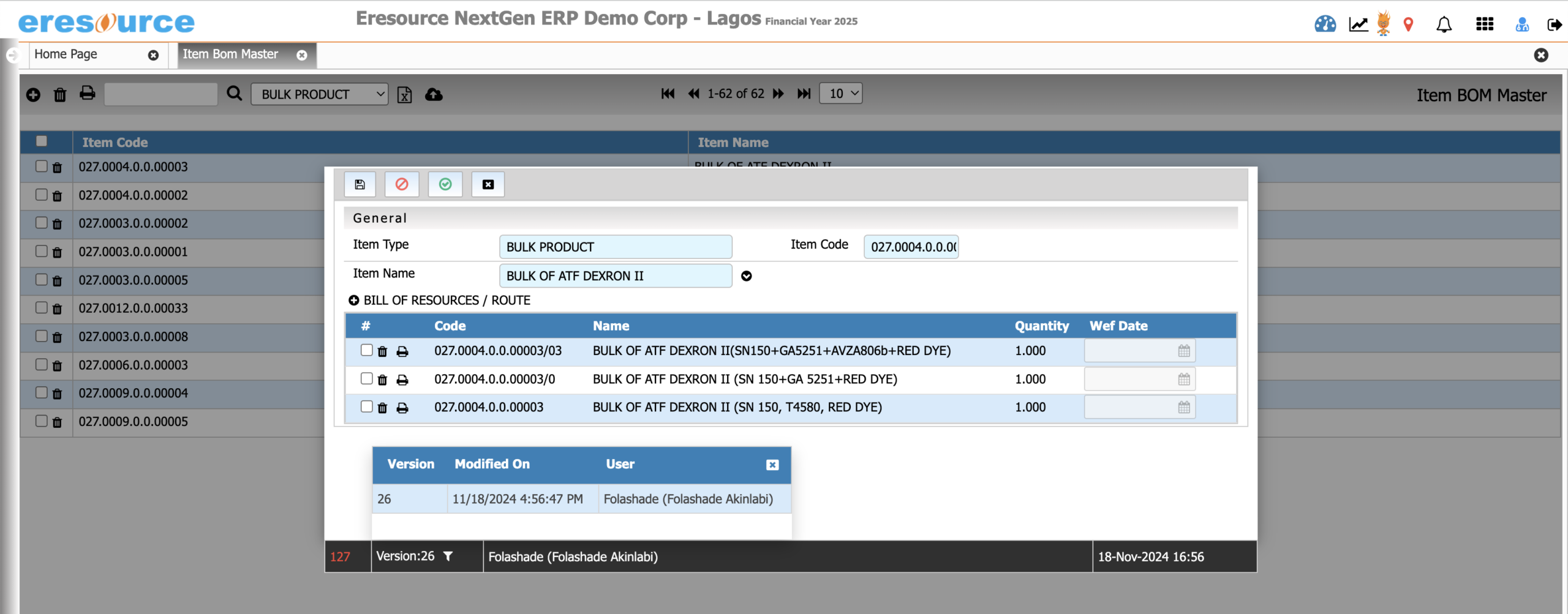
Batch Manufacturing Process
Execute batch production with complete accuracy and visibility. This module supports end-to-end batch operations and ensures compliance with regulatory requirements through detailed BMR/BPR documentation.
Key Features:
• Batch creation, scheduling, and execution
• Digital BMR and BPR tracking
• Real-time monitoring of process stages
• WIP visibility across all production points
• Auto-consumption of materials based on recipes
• Batch yield, variance, scrap, and rework recording
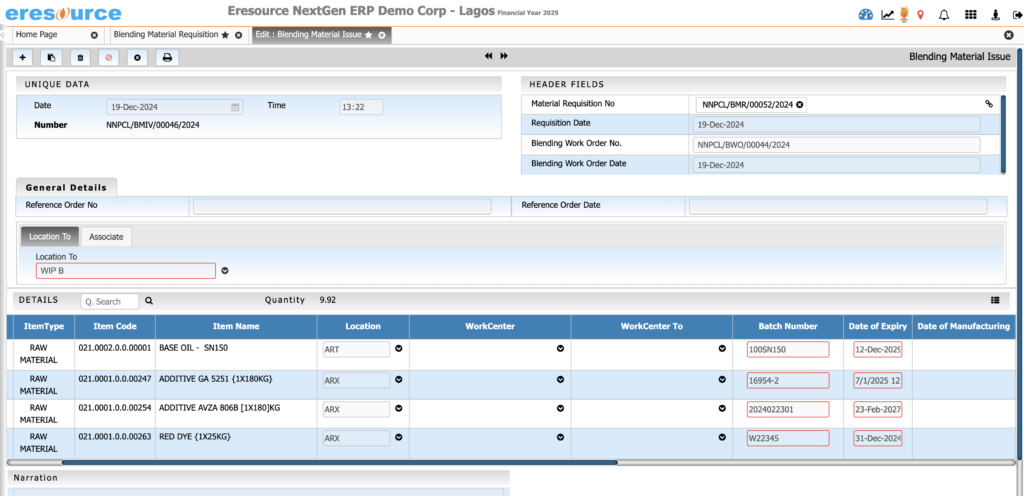
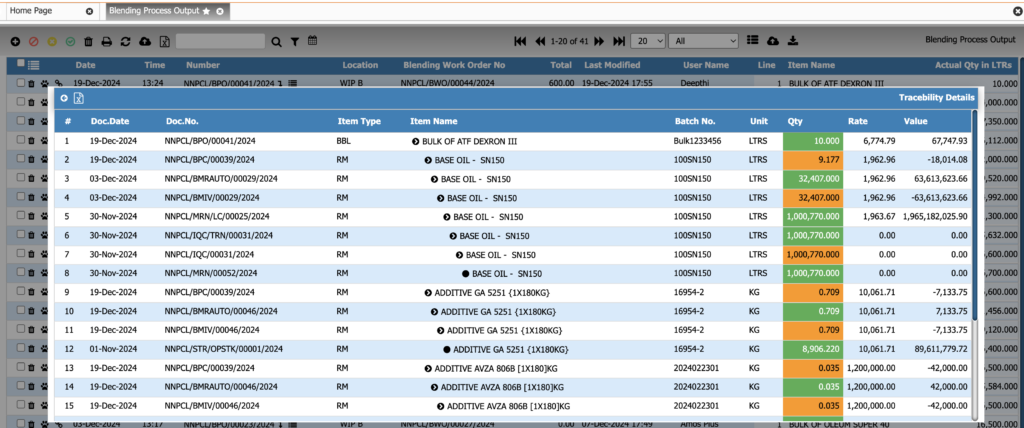
Batch Numbering & Traceability
Track every batch at every stage. This module provides full traceability from raw materials to finished products, ensuring compliance, safety, and rapid recall management.
Key Features:
• Automatic or manual batch number assignment
• Forward and backward traceability
• Complete material-to-finished-good genealogy
• Batch splitting, merging, and reclassification
• Compliance-ready traceability for pharma and food
Shelf-Life & Expiry Management
Ensure material freshness and compliance by actively managing expiry timelines. The system prevents the use of expired items and improves quality control during storage and dispatch.
Key Features:
• Automated expiry and retest date calculation
• FEFO/FIFO picking
• Alerts for near-expiry materials
• Quarantine and block controls for expired items
• Automatic restrictions during production or dispatch
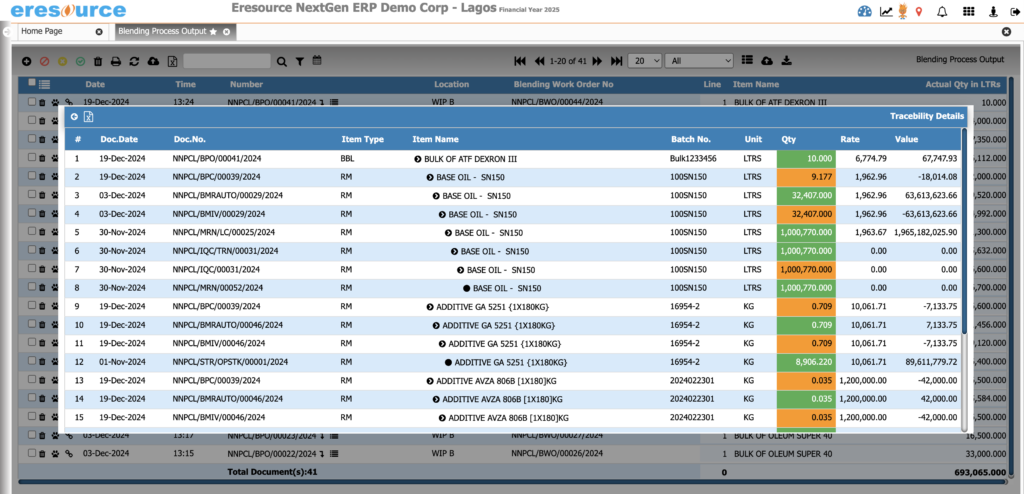
Batch Costing
Understand the true cost of every batch. This module delivers detailed costing insights that help manufacturers improve pricing decisions, reduce losses, and maximize profitability.
Key Features:
• Material, machine, labor, and overhead allocation
• Cost per batch, per unit, or per product variant
• Loss and wastage cost analysis
• Standard vs actual cost comparison
• Profitability tracking by batch or product line
Quality Control & Quality Assurance
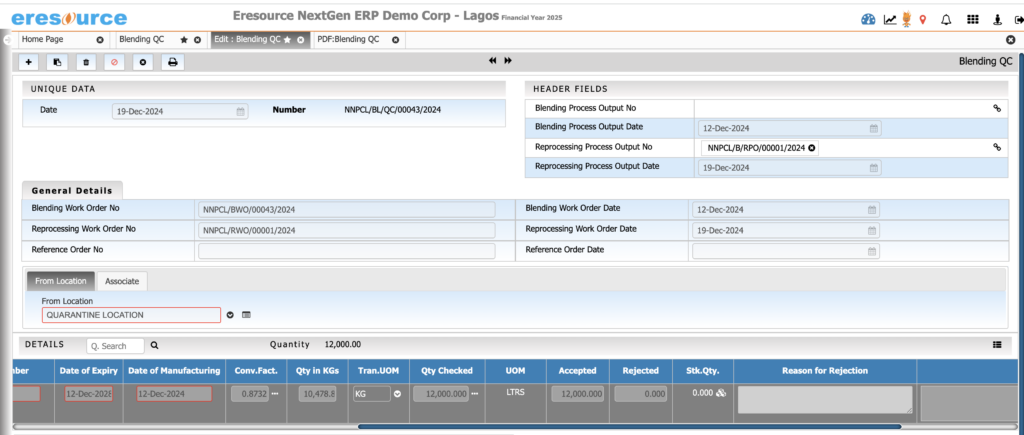
Ensure consistent product quality through structured QC and QA processes. From raw materials to finished goods, the system enforces strict checks, documentation, and compliance workflows.
a) Inward Quality Control (IQC)
- QC parameters for each raw material
- Sampling plans
- Hold, pass, and reject controls
- Vendor-wise QC performance
b) In-Process Quality Control (IPQC)
- Real-time QC at every batch stage
- Viscosity, pH, density, titration tests
- Auto-restriction if QC fails
- Deviation tracking and corrective actions
c) Finished Goods QC (FGQC)
- QC testing before packaging
- COA generation
- Batch-wise retain sample management
- QA release workflows
d) Stability Testing
- Stability study scheduling
- Condition-wise test logging
- Stability-based expiry assignment
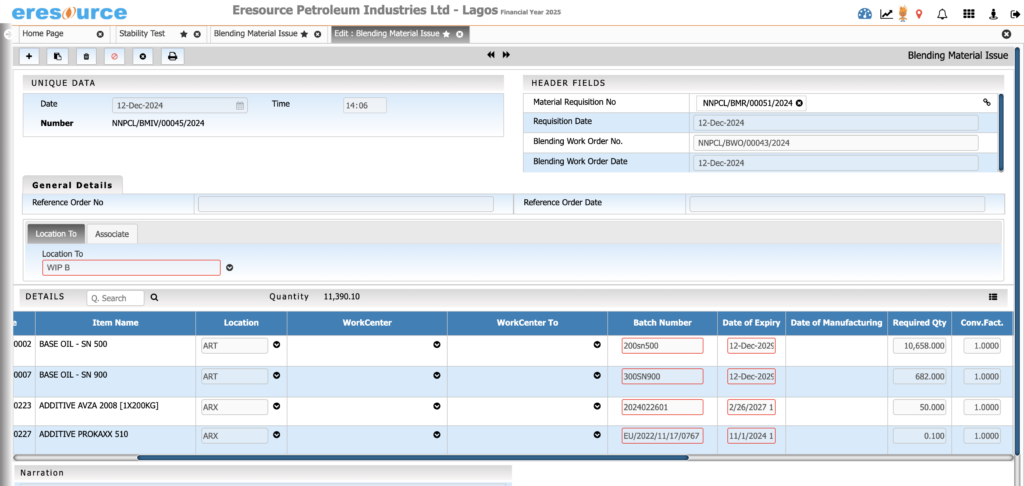
Inventory & Warehouse Management
Control your inventory with complete batch accuracy. This module ensures compliant storage, stock rotation, and material issuance for smooth and safe production cycles.
Key Features:
• Batch, grade, potency-based stock visibility
• FEFO/FIFO allocations
• Approved, rejected, and quarantine stock segregation
• Raw material issuance based on recipe
• Warehouse transfers with batch tracking
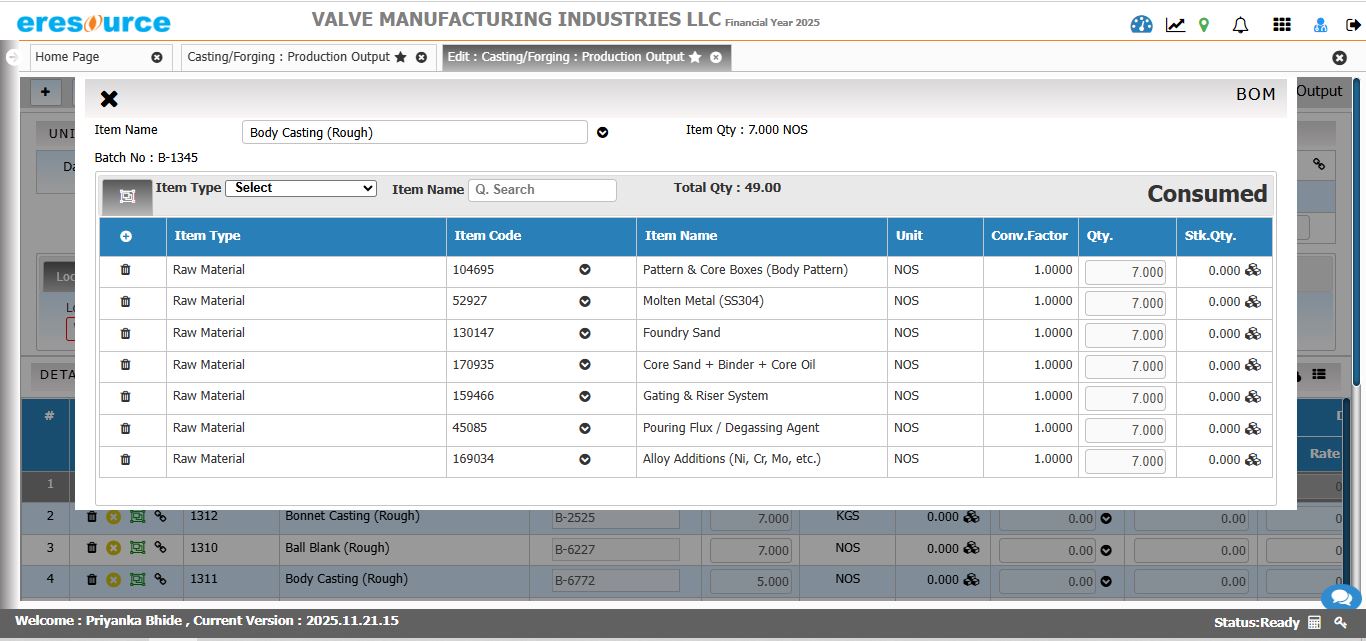
Production Planning & Scheduling
Plan production efficiently with real-time insights into capacity, availability, and demand. The system optimizes batch schedules across tanks, reactors, and processes for maximum throughput.
Key Features:
• MRP-driven raw material planning
• Reactor and vessel capacity scheduling
• Multi-level batch planning
• Automated scheduling for multi-stage processes
• Resource load balancing
Equipment & Maintenance Management
Keep production equipment running reliably. This module reduces downtime by scheduling preventive maintenance and tracking all repairs, calibrations, and associated costs.
Key Features:
• Preventive and breakdown maintenance
• Calibration and equipment validation schedules
• Maintenance work order tracking
• Cost monitoring for maintenance tasks
• Spare parts inventory
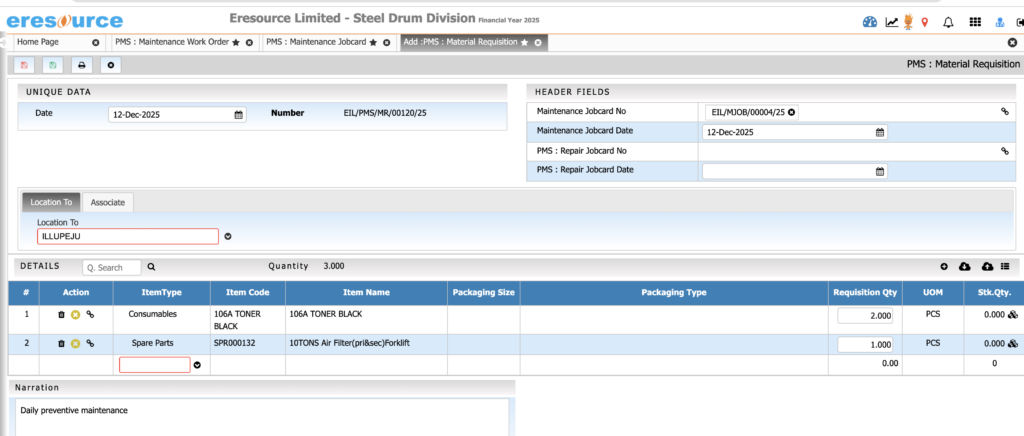
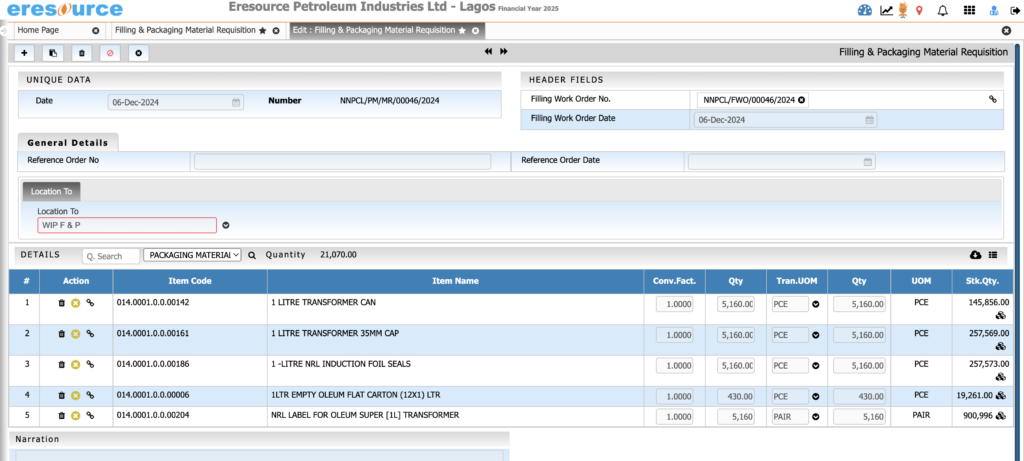
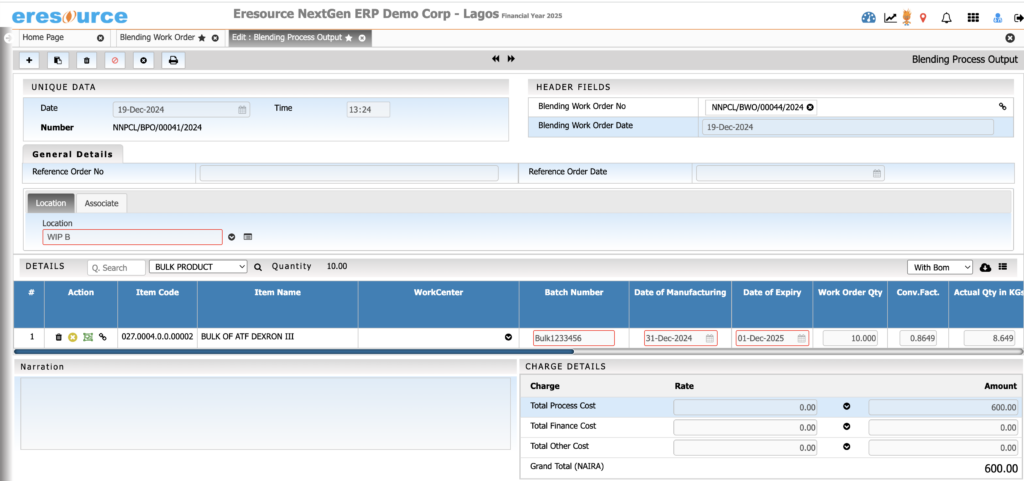
Packaging & Filling Management
Manage packaging operations seamlessly. The system supports primary and secondary packaging and ensures accurate labeling and stock deductions.
Key Features:
• Multi-stage packaging workflows
• Packaging BOM with labels, cartons, sleeves, caps
• Label printing with batch and expiry information
• Automatic consumption of packing materials
Purchase & Supplier Management
Streamline procurement with controlled and transparent purchasing processes. This module helps ensure timely availability of raw materials while maintaining vendor quality and cost efficiency.
Key Features:
• RFQ and quotation comparison
• Vendor qualification and performance tracking
• Potency-based purchase adjustments
• Purchase contracts for bulk chemicals
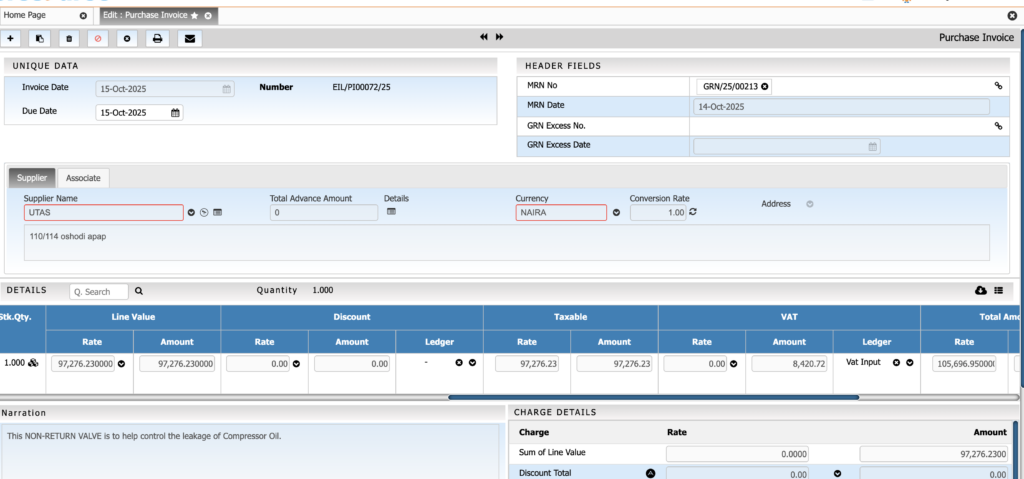
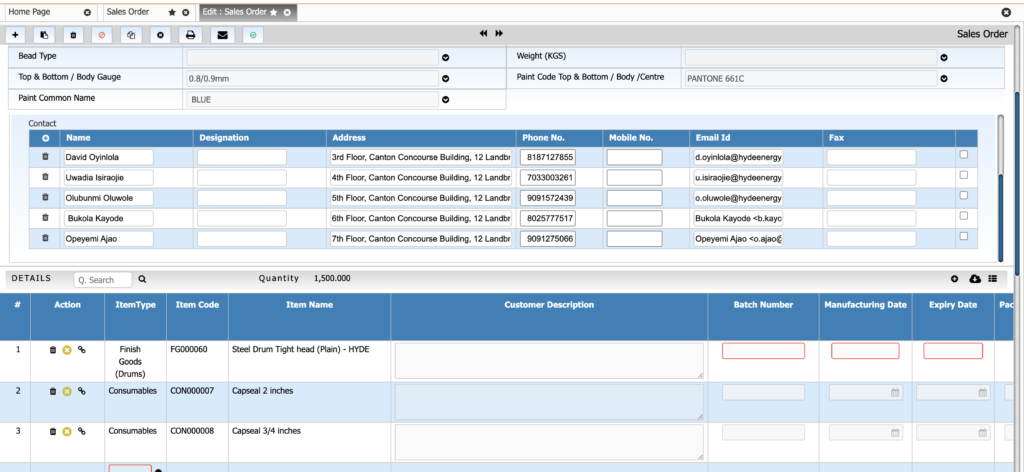
Sales & Order Management
Handle sales orders with precise control over batch selection and expiry constraints. This ensures timely dispatch and compliance with customer-specific requirements.
Key Features:
• Batch-specific order allocations
• FEFO-based batch selection
• COA attached with dispatch
• Sales forecasting linked with production
Financial Management
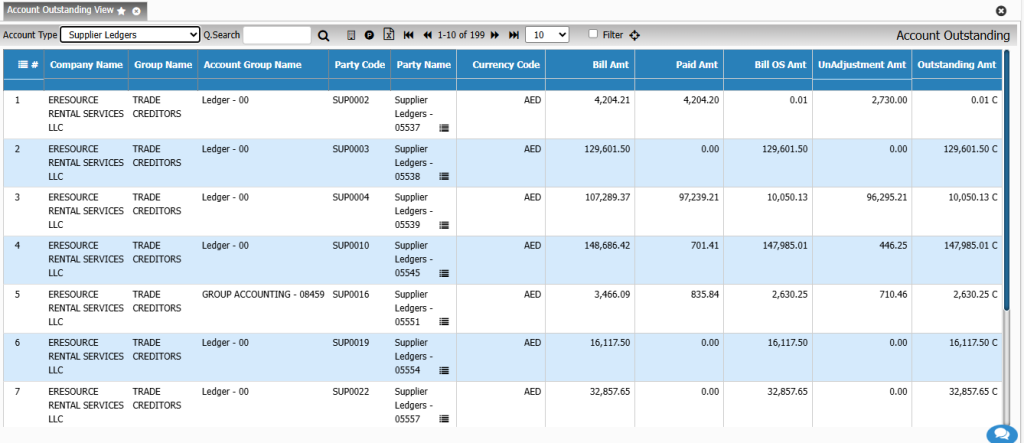
Costing & Profitability Analysis
Get a clear view of operational profitability. This module analyzes cost behavior across batches, products, and customers, enabling smarter financial decision-making.
Key Features:
• Activity-based costing
• Batch-wise cost variance
• Product profitability analysis
• Cost simulation for new formulations
Benefits for Pharmaceutical & API Manufacturers
- Maintain strict batch uniformity through controlled formulations
- Achieve full traceability across raw materials, APIs, intermediates, and final drugs
- Strengthen compliance with FDA, WHO, GMP, and Schedule M standards
- Reduce production deviations and manual documentation errors
- Improve warehouse safety with quarantine, FEFO, and controlled storage
- Enhance costing accuracy with real-time batch consumption tracking
- Increase efficiency with automated workflows and digital batch records
- Scale across multiple dosage forms and multi-plant operations
Transform the Way You Produce with eresource Bpro
eresource Bpro ERP isn’t just software, it’s a catalyst for smarter batch-based manufacturing. With precise process control, real-time visibility, and cloud-ready automation, it turns complex production workflows into smooth, predictable operations.
From formulation to batching to final output, every step becomes more consistent, efficient, and optimized for growth.
Case Studies
MediCore Pharmaceuticals
Reduced batch release time by 20% through automated electronic batch record workflows and integrated QC testing.
20% Faster Batch Release
PharmaMax Ltd
Achieved zero audit issues in recent FDA inspection with comprehensive compliance documentation and traceability.
Zero Audit Issues
API Integrations
APIs to integrate with other enterprise, compliance, finance, analytics, or planning systems as per business needs.
Real-time data exchange with LIMS, regulatory reporting systems, serialization platforms, and quality management systems.
IoT Integrations
Cold storage monitoring for temperature-sensitive materials
RFID tracking for complete batch genealogy
Cleanroom environmental sensors for GMP compliance
Video Resources
Play Video
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Play Video
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Play Video
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Play Video
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Frequently
Asked Question
Get answers to common questions about Pharmaceutical Industry

Can eresource Bpro handle formulation and master recipe management?
Yes. It manages detailed formulations, ingredient ratios, and validated master recipes.
Is the ERP compliant with FDA, GMP, WHO, and Schedule M guidelines?
Absolutely. It supports regulatory documentation, audit trails, and validation workflows.
Can it manage APIs, intermediates, and controlled raw material storage?
Yes. It supports full traceability, quarantine, FEFO, and controlled warehouse access.
Does the system support QC activities like stability and microbial tests?
Yes. All lab tests, stability records, and inspection logs are captured digitally.
Can it track granulation, compression, coating, and filling processes?
Yes. Eresource Bpro tracks each production stage with complete WIP visibility.
Is Bpro suitable for multi-plant and large-scale pharma manufacturing?
Yes. It is built for both small units and large multi-location pharmaceutical companies.
Transform Pharmaceutical Manufacturing with eresource Bpro
Experience how eresource Bpro ERP simplifies formulation, batch production, quality testing, regulatory compliance, and financial management.
